clinical trial malaysia
The health spending as a share of Gross Domestic Product GDP for the same period ranged from 295 per cent to 453 per cent of GDP. Onel enrolled 30 patients at UKM in Malaysia and runs until 2022.

Dengue Surveillance Using Gravid Oviposition Sticky Gos Trap And Dengue Non Structural 1 Ns1 Antigen Test In Malaysia Randomized Controlled Trial Scientific Reports
712A GUIDE TO CONDUCTING CLINICAL TRIALS IN MALAYSIA Fig 2.

. 32 Biomedical Research - an approach used towards solving medical problems. Malaysia is George Clinicals Southeast Asian hub where we cover neighboring countries of Singapore Indonesia and the Philippines accessing a population of almost 400 million multi-ethnic people. Clinical Trials are a process to determine the effectiveness and dangers of new medicines and other related medical practices for targeted patients.
Ad Aktuelle Jobs auf einen Blick Clinical Trial Stellenangebote finden. For more details please click here. Singapore is a recognized destination for clinical trials yet other countries are fast emerging as more.
The current Malaysian Economic Transformation Program ETP targets clinical research as one of its main drivers in economic growth. This Guideline adopts the following definitions. In most cases the smaller Asian countries will not require local clinical studies and will accept foreign clinical trial data during the registration process for both medical devices and pharmaceuticals.
31 Clinical Trial - in which the objective of the trialresearch is of essentially diagnostic or therapeutic value to the patient. What is the regulatory authority with oversight for clinical trial in Malaysia. Within 10 mi 15 km Filter By Advanced I amhavehad ct scan treatment regimen chest x-ray gaa gene movement disorder more I am looking for ergocalciferol etelcalcetide dasatinib pemetrexed adalimumab more Found 202 clinical trials By relevance.
To register new drugs applicants must also. Centre for Clinical Trial CCT was established in 2010 to conduct and support early and late phase clinical research in Malaysia. In Malaysia HIV self-testing has been shown to have moderate to high levels of acceptability depending on the population test used and test delivery framework.
Before applying for a CTC an applicant should first receive approval from the hospitals Ethics Committee EC. In the present study we aim to evaluate the acceptability and impact of an online program enabling home-based hepatitis C virus HCV self-testing in Malaysia. Hong Kong Indonesia Malaysia the Philippines Singapore Taiwan Thailand and Vietnam.
Obtaining EC approval generally takes 4 to 6 weeks. More importantly their contributions have been translated into improved patient care and outcomes and underscore the importance of evidence-based practice. For over 35 years PAREXEL has proven to be a trusted partner for the complex development journey required of biopharmaceutical and medical.
Sponsored clinical studies are usually funded by pharmaceutical companies to conduct clinical trials that involve a research treatment or therapy. A The 2007 directive requiring all ethics committees that approve clinical trials in Malaysia to be registered with the Malaysian Drug Control Authority DCA a body established under the Regulations to regulate the quality safety and efficacy of. Featured CROs in Malaysia Parexel.
Both trials are being conducted by Kumar who is. General Clinical Trial. George Clinical In Malaysia.
Since the last publication of Guideline for the application of Clinical Trial Import Licence CTIL and Clinical Trial Exemption CTX 5th Edition in 2009 we have witnessed robust growth in clinical research industry with the aim to achieve at least 1000 clinical trials to generate GNI of RM5784 million by the year 2020 in Malaysia. On August 2020 the NPRA of Malaysia has updated a document intended to guide the applicant in making Clinical Trial Import Licence CTIL and Clinical Trial Exemption CTX applications to NPRA and reporting to NPRA upon the completion of the clinical trial. List of Clinical Research Organizations in Malaysia Get Listed.
Phase I Phase I includes trials to assess the safety and effects of the medicines on body systems. Malaysian investigators have over the past decade been involved in major clinical outcome trials which were subsequently published in major medical journals. For over 20 years Malaysia has successfully gained experience in conducting clinical trials by establishing and constantly improving its facilities processes regulations and research competent staff.
The clinical trials are divided into four different clinical phases. Besides early phase clinical research we also conduct bioavailability and bioequivalence studies for pharmaceutical industries in accordance to local and international standards. US Clinical Trials Registry.
The Total Health Expenditure THE for Malaysia during 1997-2013 ranged from RM8303 million in 1997 to RM44748 million in 2013. The Centre for Investigational New Product is the unit in charge. Following the failure Moleac engaged Kumar for further trials to find alternative uses for the drug.
A listing of Kuala Lumpur Malaysia clinical trials actively recruiting patients volunteers. A clinical trial conducted according to a single protocol but at more than one site and therefore carried out by more than one investigator. EU Clinical Trials Registry.
Malaysian Guideline for Application of Clinical Trial Import Licence and Clinical Trial Exemption National Pharmaceutical Control Bureau Ministry of Health Malaysia Malaysian Guideline for Application of CTIL and CTX National Pharmaceutical Control Bureau ii. National Committee for Clinical Research A committee established for the purpose of coordinating and promoting clinical research in Malaysia chaired by the Director General of Health Ministry of Health. A second has enrolled 2000 patients at UKM and the National Brain Center Hospital in Indonesia and is to run until January 2022.
Malaysia has a single regulatory authority the National Pharmaceutical Control Bureau NPCB. Under Regulation 36B of the Pharmacy and Poisons Regulations a certificate for Clinical TrialMedicinal Test CTC is required before conducting a clinical trial. In Malaysia sponsored research has been conducted in 220 clinical study sites since 2012 involving Ministry of Health MOH facilities university hospitals and private medical centres.
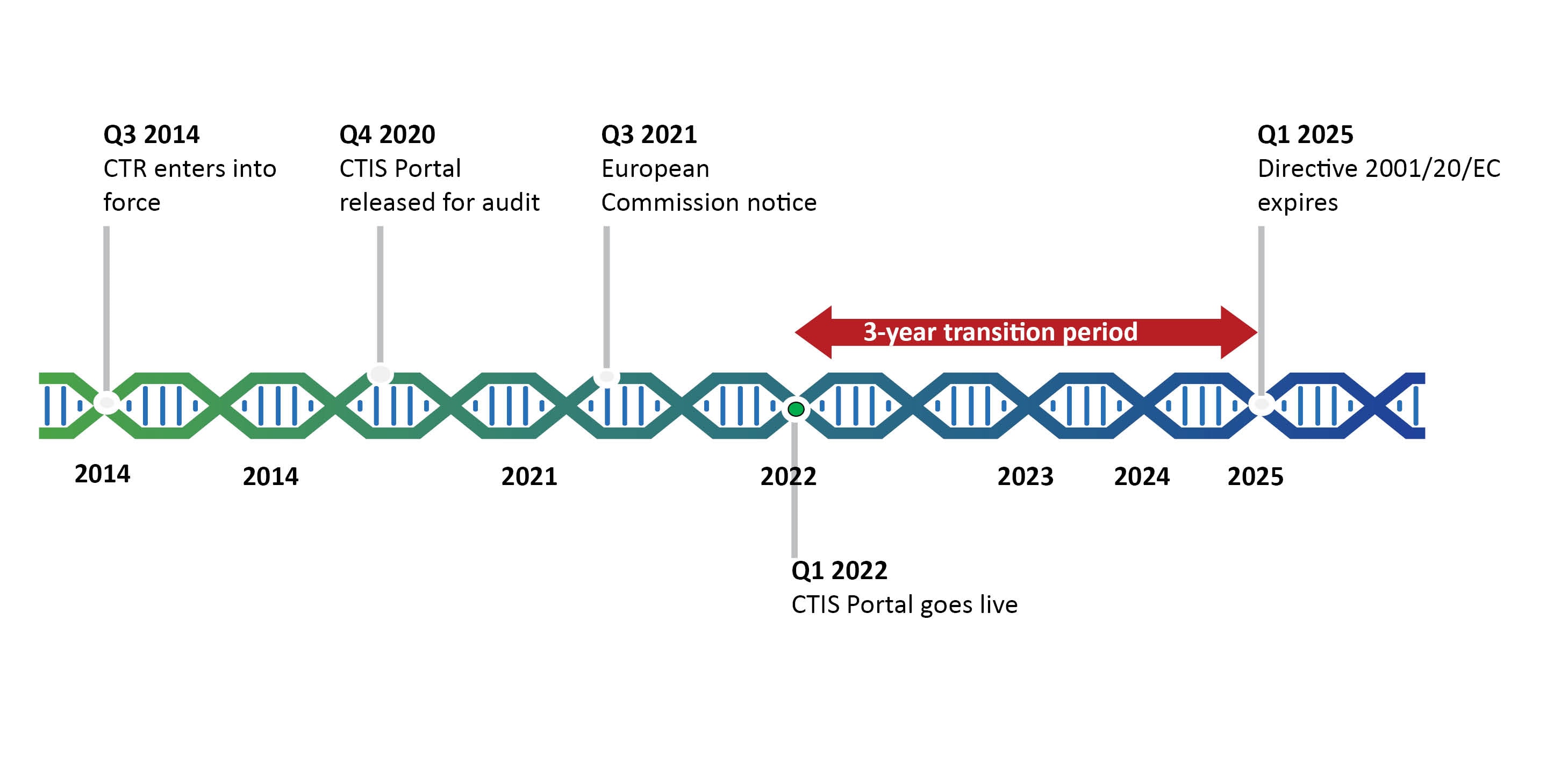
Introduction To The Clinical Trials Regulation Deloitte Netherlands

The Progression Of Clinical Trials In Indonesia An Observational Study Of Records From Clinical Trial Registries Databases Sciencedirect

National Medical Research Register

Asia Pacific Apac Cro Services Precision For Medicine
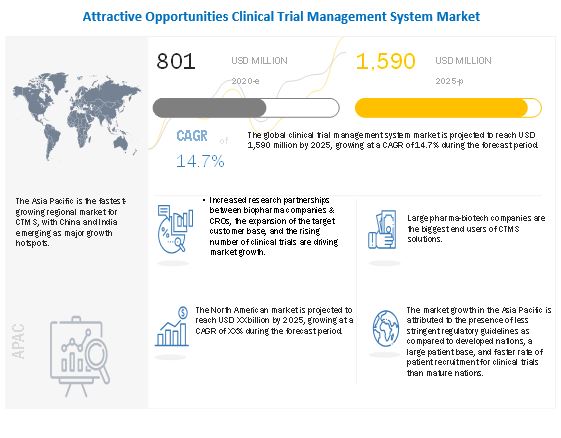
Clinical Trial Management System Market 2022 2025 Size Share And Trends Marketsandmarkets
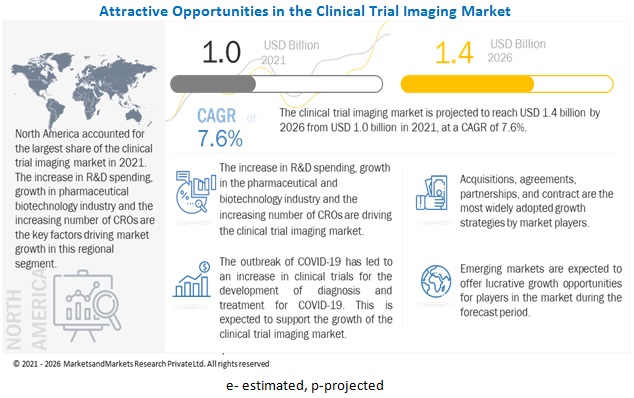
Clinical Trial Imaging Market Global Forecast To 2026 Marketsandmarkets

Institute For Clinical Research Icr Nih My Icr Nih Twitter

Institute For Clinical Research Icr Nih My Icr Nih Twitter

The Magic Glasses Philippines A Cluster Randomised Controlled Trial Of A Health Education Package For The Prevention Of Intestinal Worm Infections In Schoolchildren The Lancet Regional Health Western Pacific

Home Clinical Update In Covid 19
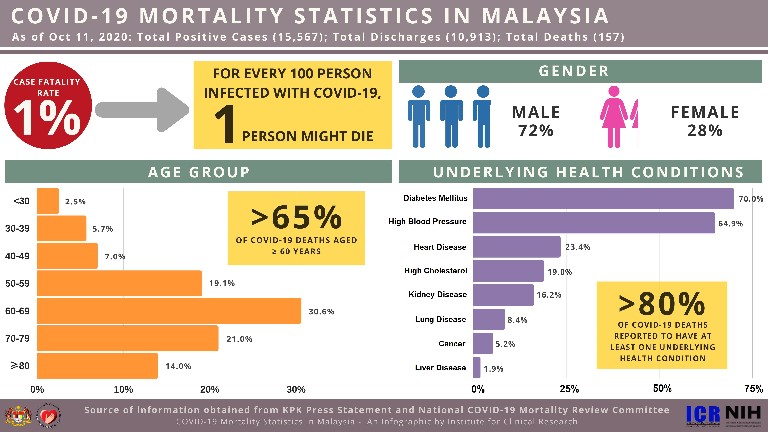
Nih 70 Of Malaysia S Coronavirus Fatalities Had Diabetes Codeblue
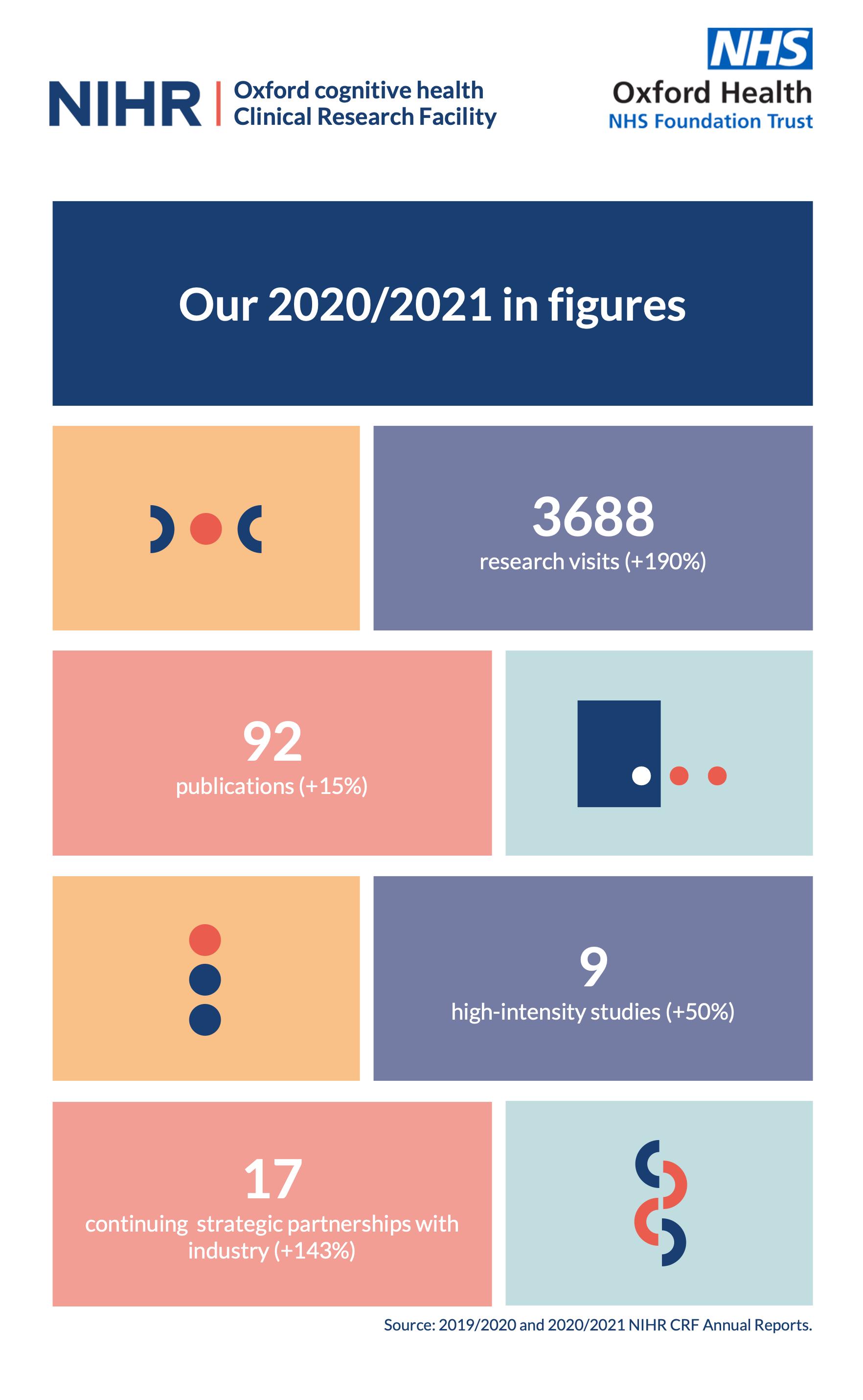
Nihr Oxford Cognitive Health Clinical Research Facility Nihr Oxford Health Biomedical Research Centre

Ministry Of Health Malaysia And Drugs For Neglected Diseases Initiative Combine Forces To Lead The Battle Against Dengue Dndi

Clinical Research Services For Early Stage Biotech Companies

The Progression Of Clinical Trials In Indonesia An Observational Study Of Records From Clinical Trial Registries Databases Sciencedirect
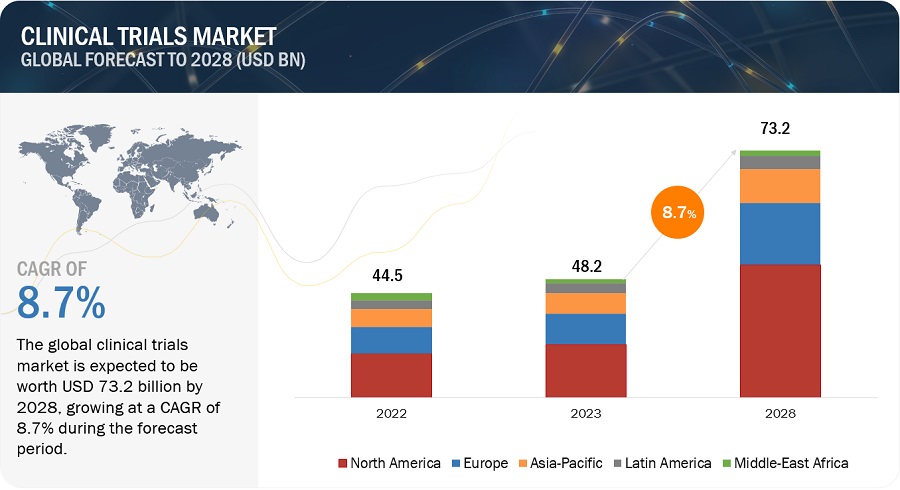
Clinical Trials Market Size Share 2022 2026 Marketsandmarkets

Clinical Research Services For Early Stage Biotech Companies


Comments
Post a Comment